Cell-Cultured Meat
The process begins by obtaining a small sample of animal cells, typically derived from livestock, and
then nurturing them in a controlled laboratory environment. Primary cells can be acquired by tissue
isolation from a donor animal, which can subsequently be used to produce a cell bank suitable for
cultivated meat. These cells can be immortalized with gene editing technologies such as CRISPR.
Embryonic stem cells, adult stem cells (i.e., mesenchymal stem cells, MSCs), or induced pluripotent
stem cells (iPSCs) can also be utilized, which can be directed into the desired cell types for the meat
product. Over time, these cells grow and develop into authentic meat that is biologically identical to
conventionally produced meat, both in terms of composition and taste.
As exciting as cell-cultured meat is, ensuring its safety is of paramount importance. Adventitious agents, such as bacteria, viruses, and other microorganisms, can pose serious health risks if present in the final product.
ViruSure, with over two decades of experience in biosafety testing and virus clearance, is your
knowledgeable partner for testing your cultured meat product.
How can ViruSure support you?
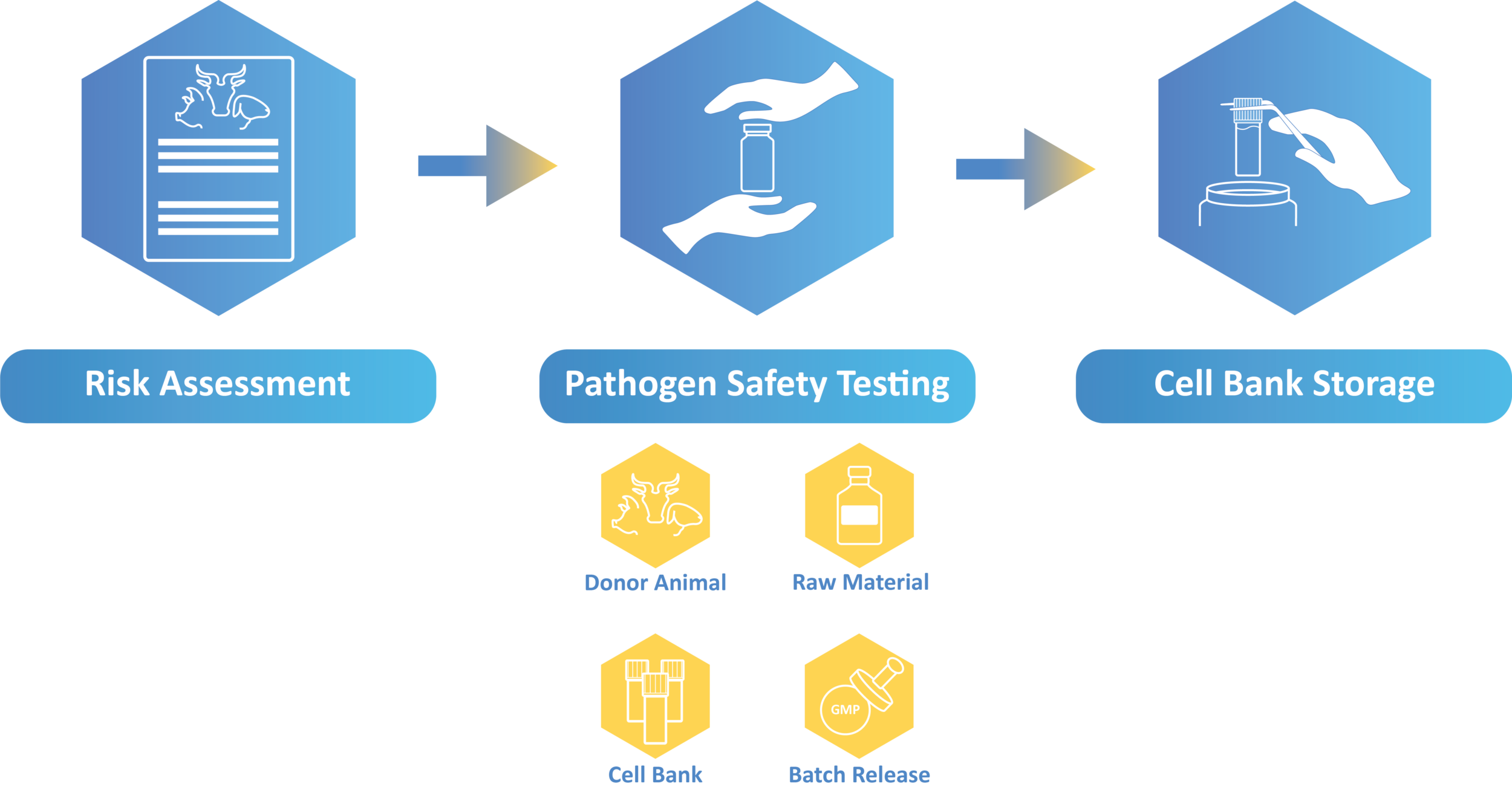
- Risk Assessments: We can support you with risk assessment considering the geographical
origin of the donor animals, veterinary surveillance, and possible zoonosis. - Donor Animals Testing: The testing is performed according to the risk assessment.
- Cell Bank Testing: The testing panel will depend on the technology used for the generation of
the bank. For a tailored testing package, contact us. - Raw Material Testing: Raw materials can be used during the generation of the cell line as well
as during the manufacturing process. For cell line generation, FBS and porcine trypsin are often used with additional risk from a pathogen safety perspective. For manufacturing, even when xeno-free materials are used, the risk of contaminants should be considered. - Batch Release: Mycoplasma and sterility testing
- Long term Storage: Our GMP-compliant storage area offers cryostorage storage options.
Which of our services are applicable for cell cultured meat products?
Our GMP-certified, state-of-the-art facilities and highly trained scientific experts ensure that your cultured meat product will meet the highest standards of safety. The testing panel for the cell bank is highly depends on the technology used to produce cultivated meat (e.g., stem cells, iPSCs, gene editing, viral vectors or immortalized cell lines). For a customized testing panel, please reach out to our experts.
ViruSure offers the following services
Molecular Tests (e.g. qPCR for human, bovine, avian, or equine viruses)
Genetic Sequencing (Sanger sequencing & NGS)
